OBSERVE & ASK
The first is an examination procedure that provides instructions to systematically observe and ask about movements in patients taking APDs.
AIMS is a 12-item clinician-rated exam for TD and the standard of care to:
AIMS consists of 2 parallel processes:
The first is an examination procedure that provides instructions to systematically observe and ask about movements in patients taking APDs.
The second process is scoring the severity of observed movements on a 12-item scoring card.
Patient images used with permission.
Click on each AIMS Procedure Step to watch a video highlighting the associated movement.
Patient images used with permission.
STEP 1
Ask the patient: Is there anything in your mouth?
If yes, ask the patient to remove it.
STEP 2
Ask the patient: Do you wear dentures? Do your teeth or dentures bother you now?
STEP 3
Ask the patient: Do you notice any abnormal movements in your mouth, face, hands, or feet?
If yes, ask the patient to describe the movements and how bothersome they are.
STEP 4
Have the patient sit in the chair with hands on knees, legs slightly apart, and feet flat on the floor.
Observe the entire body and rate any abnormal movements.
STEP 5
Ask the patient to sit with hands hanging and unsupported between the legs or over the knees.
Observe hands and other body areas.
STEP 6
Ask the patient to open his or her mouth. Do this twice.
Observe the tongue at rest.
STEP 7
Ask the patient to protrude his or her tongue. Do this twice.
Observe abnormalities of tongue movements.
STEP 8
Ask the patient to tap his or her thumb with each finger as rapidly as possible for 10 to 15 seconds, first with the right hand, then with the left hand.
Observe the face and legs.
STEP 9
Flex and extend the patient’s left and right arms, one at a time.
Note any rigidity or muscle stiffness that could indicate the potential presence of parkinsonism.
STEP 10
Ask the patient to stand, arms to the side, so they can be observed in profile and straight on. Observe for truncal movements.
STEP 11
Ask the patient to extend both arms out in front, palms down.
Observe the face and legs.
STEP 12
Have the patient walk a few paces, turn, and walk back to the chair. Do this twice.
Observe hands and gait.
| MOVEMENT RATINGS | SCORE | ||
|---|---|---|---|
| Facial & Oral Movements |
1.
Muscles of facial expression |
01234 | |
| 2.
Lips and perioral area |
01234 | ||
| 3.
Jaw |
01234 | ||
| 4.
Tongue |
01234 | ||
| Extremity Movements |
5.
Upper (arms, wrists, hands, fingers) |
01234 | |
| 6.
Lower (legs, knees, ankles, toes) |
01234 | ||
| Trunk Movements | 7.
Neck, shoulders, hips |
01234 | |
| Global Judgments |
8.
Severity of abnormal movements overall |
01234 | |
| 9.
Incapacitation due to abnormal movements |
01234 | ||
| 10.
Patient awareness of abnormal movements |
01234 | ||
| Dental Status | 11.
Current problems with teeth/dentures? |
NoYes | |
| 12.
Are dentures usually worn? |
NoYes | ||
Each of the first 7 items
Measures specific areas of the body
Is scored on a 0-to-4 scale
Depends on the frequency of the movements and how easy they are to detect
| MOVEMENT RATINGS | SCORE | ||
|---|---|---|---|
| Facial & Oral Movements |
1.
Muscles of facial expression |
01 |
|
| 2.
Lips and perioral area |
0123 |
||
| 3.
Jaw |
01 |
||
| 4.
Tongue |
0123 |
||
| Extremity Movements |
5.
Upper (arms, wrists, hands, fingers) |
012 |
|
| 6.
Lower (legs, knees, ankles, toes) |
01 |
||
| Trunk Movements | 7.
Neck, shoulders, hips |
01 |
|
| Global Judgments |
8.
Severity of abnormal movements overall |
0123 |
|
| 9.
Incapacitation due to abnormal movements |
01234 | ||
| 10.
Patient awareness of abnormal movements |
01234 | ||
| Dental Status | 11.
Current problems with teeth/dentures? |
NoYes | |
| 12.
Are dentures usually worn? |
NoYes | ||
Item 8 assesses the overall severity of movements. This score is based on the highest single score in items 1 to 7.
| MOVEMENT RATINGS | SCORE | ||
|---|---|---|---|
| Facial & Oral Movements |
1.
Muscles of facial expression |
01 |
|
| 2.
Lips and perioral area |
0123 |
||
| 3.
Jaw |
01 |
||
| 4.
Tongue |
0123 |
||
| Extremity Movements |
5.
Upper (arms, wrists, hands, fingers) |
012 |
|
| 6.
Lower (legs, knees, ankles, toes) |
01 |
||
| Trunk Movements | 7.
Neck, shoulders, hips |
01 |
|
| Global Judgments |
8.
Severity of abnormal movements overall |
0123 |
|
| 9.
Incapacitation due to abnormal movements |
0123 |
||
| 10.
Patient awareness of abnormal movements |
012 |
||
| Dental Status | 11.
Current problems with teeth/dentures? |
NoYes | |
| 12.
Are dentures usually worn? |
NoYes | ||
Items 9 and 10 assess the impact of TD and may be useful in clinical decision-making.
| MOVEMENT RATINGS | SCORE | ||
|---|---|---|---|
| Facial & Oral Movements |
1.
Muscles of facial expression |
01 |
|
| 2.
Lips and perioral area |
0123 |
||
| 3.
Jaw |
01 |
||
| 4.
Tongue |
0123 |
||
| Extremity Movements |
5.
Upper (arms, wrists, hands, fingers) |
012 |
|
| 6.
Lower (legs, knees, ankles, toes) |
01 |
||
| Trunk Movements | 7.
Neck, shoulders, hips |
01 |
|
| Global Judgments |
8.
Severity of abnormal movements overall |
0123 |
|
| 9.
Incapacitation due to abnormal movements |
0123 |
||
| 10.
Patient awareness of abnormal movements |
012 |
||
| Dental Status | 11.
Current problems with teeth/dentures? |
No |
|
| 12.
Are dentures usually worn? |
No |
||
Items 11 and 12 assess dental issues.
| MOVEMENT RATINGS | SCORE | ||
|---|---|---|---|
| Facial & Oral Movements |
1.
Muscles of facial expression |
01 |
|
| 2.
Lips and perioral area |
0123 |
||
| 3.
Jaw |
01 |
||
| 4.
Tongue |
0123 |
||
| Extremity Movements |
5.
Upper (arms, wrists, hands, fingers) |
012 |
|
| 6.
Lower (legs, knees, ankles, toes) |
01 |
||
| Trunk Movements | 7.
Neck, shoulders, hips |
01 |
|
| Global Judgments |
8.
Severity of abnormal movements overall |
0123 |
|
| 9.
Incapacitation due to abnormal movements |
012 |
||
| 10.
Patient awareness of abnormal movements |
0123 |
||
| Dental Status | 11.
Current problems with teeth/dentures? |
No |
|
| 12.
Are dentures usually worn? |
No |
||
The AIMS total score is determined by adding items 1 to 7.
AIMS total score (sum of 1 to 7)
is 12.
A minimum score of 2 on any AIMS item from 1 to 7 means that TD movements have been clearly identified and documented, requiring additional assessment of impact.

Question 1 of
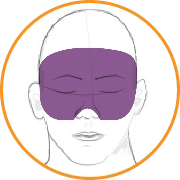
In this video, select the patient’s score for AIMS item 1: Muscles of facial expression.
Patient image used with permission.
Answer: 1
Symptoms are minimal (ie, abnormal movements occur infrequently and/or are difficult to detect).
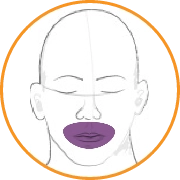
In this video, select the patient’s score for AIMS item 2: Lips and perioral area.
Patient image used with permission.
Answer: 4
Symptoms are severe (ie, abnormal movements occur almost continuously and/or are of extreme intensity).
In this video, select the patient’s score for AIMS item 3: Jaw (eg, biting, clenching, chewing, mouth opening, lateral movement).
Patient image used with permission.
Answer: 3
Symptoms are moderate (ie, abnormal movements occur frequently and/or are easy to detect).

In this video, select the patient’s score for AIMS item 4: Tongue (look for movements in the tongue and rate only writhing or twisting movements that occur either in or out of the mouth).
Patient image used with permission.
Answer: 2
Symptoms are mild (ie, abnormal movements occur infrequently and/or are easy to detect).
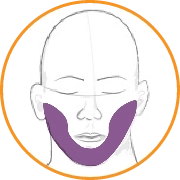
In this video, select the patient’s score for AIMS item 7: Neck, shoulders, hips.
Patient image used with permission.
Answer: 3
Symptoms are moderate (ie, abnormal movements occur frequently and/or are easy to detect).
Thank you for completing
Screening and Assessment: Chapter 2
Abnormal Involuntary Movement Scale (AIMS):
Standard of Care for the Screening and Assessment of TD
Summary:
AIMS is part of the standard of care for assessing and monitoring TD.
Chapter 2 References:
American Psychiatric Association. The American Psychiatric Association Practice Guideline for the Treatment of Patients With Schizophrenia. 3rd ed. Washington, DC: American Psychiatric Association; 2021.
Data on file. Teva Neuroscience, Inc. Parsippany, NJ.
Guy W. ECDEU Assessment Manual for Psychopharmacology: Revised. Rockville, MD; 1976:534-537.
Lane RD et al. J Nerv Ment Dis. 1985;173(6):353-357.
Munetz MR, Benjamin S. Hosp Community Psychiatry. 1988;39(11):1172-1177.
STABLE National Coordinating Council Resource Toolkit Workgroup. https://www.scribd.com/document/501791293/STABLE-Resource-Toolkit-Bipolar.
AUSTEDO XR® (deutetrabenazine) extended-release tablets and AUSTEDO® (deutetrabenazine) tablets are indicated in adults for the treatment of chorea associated with Huntington’s disease and for the treatment of tardive dyskinesia.
Depression and Suicidality in Patients with Huntington’s Disease: AUSTEDO XR® and AUSTEDO® can increase the risk of depression and suicidal thoughts and behavior (suicidality) in patients with Huntington’s disease. Balance the risks of depression and suicidality with the clinical need for treatment of chorea. Closely monitor patients for the emergence or worsening of depression, suicidality, or unusual changes in behavior. Inform patients, their caregivers, and families of the risk of depression and suicidality and instruct them to report behaviors of concern promptly to the treating physician. Exercise caution when treating patients with a history of depression or prior suicide attempts or ideation. AUSTEDO XR and AUSTEDO are contraindicated in patients who are suicidal, and in patients with untreated or inadequately treated depression.
Contraindications: AUSTEDO XR and AUSTEDO are contraindicated in patients with Huntington’s disease who are suicidal, or have untreated or inadequately treated depression. AUSTEDO XR and AUSTEDO are also contraindicated in: patients with hepatic impairment; patients taking reserpine or within 20 days of discontinuing reserpine; patients taking monoamine oxidase inhibitors (MAOIs), or within 14 days of discontinuing MAOI therapy; and patients taking tetrabenazine or valbenazine.
Clinical Worsening and Adverse Events in Patients with Huntington’s Disease: AUSTEDO XR and AUSTEDO may cause a worsening in mood, cognition, rigidity, and functional capacity. Prescribers should periodically re-evaluate the need for AUSTEDO XR or AUSTEDO in their patients by assessing the effect on chorea and possible adverse effects.
QTc Prolongation: AUSTEDO XR and AUSTEDO may prolong the QT interval, but the degree of QT prolongation is not clinically significant when AUSTEDO XR or AUSTEDO is administered within the recommended dosage range. AUSTEDO XR and AUSTEDO should be avoided in patients with congenital long QT syndrome and in patients with a history of cardiac arrhythmias.
Neuroleptic Malignant Syndrome (NMS), a potentially fatal symptom complex reported in association with drugs that reduce dopaminergic transmission, has been observed in patients receiving tetrabenazine. The risk may be increased by concomitant use of dopamine antagonists or antipsychotics. The management of NMS should include immediate discontinuation of AUSTEDO XR and AUSTEDO; intensive symptomatic treatment and medical monitoring; and treatment of any concomitant serious medical problems.
Akathisia, Agitation, and Restlessness: AUSTEDO XR and AUSTEDO may increase the risk of akathisia, agitation, and restlessness. The risk of akathisia may be increased by concomitant use of dopamine antagonists or antipsychotics. If a patient develops akathisia, the AUSTEDO XR or AUSTEDO dose should be reduced; some patients may require discontinuation of therapy.
Parkinsonism: AUSTEDO XR and AUSTEDO may cause parkinsonism in patients with Huntington’s disease or tardive dyskinesia. Parkinsonism has also been observed with other VMAT2 inhibitors. The risk of parkinsonism may be increased by concomitant use of dopamine antagonists or antipsychotics. If a patient develops parkinsonism, the AUSTEDO XR or AUSTEDO dose should be reduced; some patients may require discontinuation of therapy.
Sedation and Somnolence: Sedation is a common dose-limiting adverse reaction of AUSTEDO XR and AUSTEDO. Patients should not perform activities requiring mental alertness, such as operating a motor vehicle or hazardous machinery, until they are on a maintenance dose of AUSTEDO XR or AUSTEDO and know how the drug affects them. Concomitant use of alcohol or other sedating drugs may have additive effects and worsen sedation and somnolence.
Hyperprolactinemia: Tetrabenazine elevates serum prolactin concentrations in humans. If there is a clinical suspicion of symptomatic hyperprolactinemia, appropriate laboratory testing should be done and consideration should be given to discontinuation of AUSTEDO XR and AUSTEDO.
Binding to Melanin-Containing Tissues: Deutetrabenazine or its metabolites bind to melanin-containing tissues and could accumulate in these tissues over time. Prescribers should be aware of the possibility of long-term ophthalmologic effects.
Common Adverse Reactions: The most common adverse reactions for AUSTEDO (>8% and greater than placebo) in a controlled clinical study in patients with Huntington’s disease were somnolence, diarrhea, dry mouth, and fatigue. The most common adverse reactions for AUSTEDO (4% and greater than placebo) in controlled clinical studies in patients with tardive dyskinesia were nasopharyngitis and insomnia. Adverse reactions with AUSTEDO XR extended-release tablets are expected to be similar to AUSTEDO tablets.
Please see accompanying full Prescribing Information, including Boxed Warning.